The US and the UK Master’s Part / Chapter-wise Dissertation Writing Service

Then You’ve Certainly Reached the Right place
Carbon Nanotubes
Description
The paper indicates how carbon nanotubes now play an intrinsic part in nanotechnology. It also indicates how carbon nanotubes can be fabricated, synthesized and also presents an overview of its limitations and steps to overcome the limitations. The paper presents an in-depth look into the properties of nanotubes and substantiates it with the use of diagrams and equations. In addition, the paper presents an in-depth outlook into the use of titanium oxide nanotubes as implants in the nanotechnology domain. It outlines the procedures that need to be undertaken to produce titanium oxide nanotubes with the use of electrodes.Keywords: carbon atoms, nanotubes, titanium oxide nanotubes, CNTs, nanotechnology, heat stability, fabrication, applications
Arrays of carbon atoms that are hexagonal in shape are turned into tubes and these thin cylinders made of purified carbon are used to make transistors and wires as they exhibit electrical properties. The development of the carbon nanotubes (CNTs) can be called as a developmental success of the field of nanotechnology that involves the constitution of electronic circuits from one molecule or atom. There are a lot of hypothetical potentials put forward for the tremendous utility of the carbon nanotubes but these are yet to materialize. The carbon nanotubes have recently been found to emit electrons on induction by light which in turn has led to the exciting possibility that they could be used in the making of the solar cells. Thus they show intriguing possibilities to revolutionize the field of X-rays by replacing the metal filament that tend to burn out rapidly. This essay provides an overview of why carbon nanotubes are important field emitters.
Synthesis of CNTs
They are synthesized on a substrate of silica that measures about 10 mm × 10 mm at a temperature of about 900 degree celcius using a specially designed apparatus. Thus a ferrocene solution in the solvent cyclohexane that has 2 % by weight served both the purposes of carbon provider as well as a catalyst. The layer of carbon nanotube exhibited a thickness of 0.3 millimetre and weighed 3 milli grams. It is confirmed from raman spectroscopy that the material of carbon is actually homogenous and the technique of x-ray diffraction affirms that the morphological aspects are also reproducible. Okotrub et.al. (2009).CNTs as field emitters
It would not be an overstatement if we say that the next generation of cold cathode substance would use electron field emitters based on carbon nanotubes (CNTs). It is especially relevant to the field of flat-panel field-emission displays (FEDs). There are many properties that make the carbon nanotubes the preferable choice for field emission like “high aspect ratio, high chemical stability, high thermal conductivity, and high mechanical strength”.Methods for fabricating CNTs
Lot of investigations have been done to develop appropriate method s for the fabrication of the field emitters. There are basically two types of methods employed. They are
- The method of direct growth
- Method of screen printing
The method of direct growth has many disadvantages that include loss of control over individual CNT, the complicated nature of the process of fabrication, and the extremely high temperature employed for the growth. But the method of screen printing is a relatively simplistic procedure as it is found to be greatly helpful in the mass production processes. Sung M. (2006)
Limitations and means to overcome
But there are a lot of limitations associated with this method technology wise that further limits the practical applications of this process. They include: lack of proper adhesion of the carbon nanotube powders with the substrate materials, lack of homogenous and uniform distribution of the carbon nanotube powders, less degree of reproducibility because of the vast array of steps involved in the production processes of the carbon nanotubes and also a relatively less life time.
A comparatively consistent distribution can be brought about by the method of electrophoresis. As the bonding between the CNT and the substrate is not strong, a charge on the surface of the CNT is generated by the means of a detergent or charger. In some cases both may be combined and used in suspension for providing the needed charge. The function of the detergent is that it acts as a supplier of charge as well as the medium of dispersion so as to create a suspension of carbon nanotube as a result. The tail portion of the detergent is usually hydrophobic which by means of adsorption attaches to the surface area of the carbon nanotubes through interactions that are hydrophobic in nature. Thus the detergent covered CNT would exhibit some charges on the surface due to the water attracting tail of the detergent. This will be attracted by a field of electricity. This tremendous use of the detergent after subjecting to the process of electrophoresis has not been viewed with great interest and the production processes tend to completely eliminate the detergent as it was supposed to minimize field emission because of its properties of insulation. But in turn the detergent has proved to be an “organic binder” that enhances the bonding between the carbon nanotubes and the substrate. Further due to the use of this method the Carbon nanotubes can be made to have a clean surface. Sung M. (2006)
Deposition by electrophoresis and formation of fissures
A tiny film of carbon nanotubes on a substrate of titanium were deposited from a watery mix of carbon nanotubes by the method of Sodium dodecyl sulphate (SDS) electrophoresisSung (2006). A small coating of SDS was further deposited by electrophoresis. The coating was then made dry by natural means and the process of compression was used to make the surface smooth Choi.(2001).
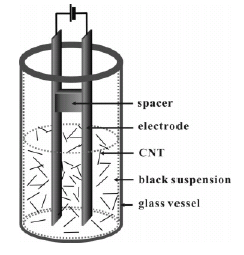
Electrophoresis process for the selective deposition of CNTs.
(Adapted from Sung et al (2006))
On firing the material at the desired temperature, the tiny film of material was altered to produce a abundant micro-tiny islands of films and thus the carbon noanotubes possessing clean surfaces were shown in the fissures that were formed. The extra deposit of SDS on the surface of the carbon nanotube potentially developed the properties of emission of the filter present in the carbon nanotube. Further, by the process of cutting, the “field emission current density” further improved. But a drawback is that the stability was minimized. This method offers the following advantages like simplicity and clean tips Gao.(2001). Also another favorable point is that the carbon nanotube soot could be used without further processes of purification and to enhance the field emission properties no additional treatments are necessary. This method also is in agreement with the Fowler-Nordheim (FN) equation Fowler (1928).
Why CNT transfer technology
In the case of applications like the electronic devices the method of “chemical vapor deposition (CVD)” are potentially useful as it possesses features of growth like “selective spatial growth, large area deposition capability, and aligned CNT growth”.
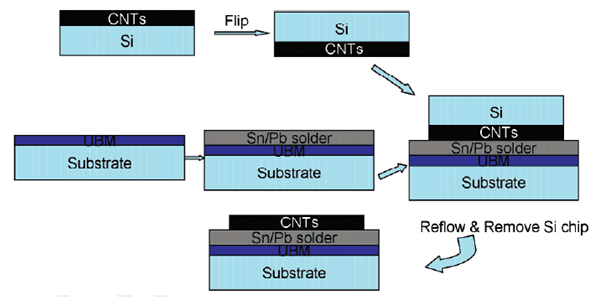
Schematic diagram of “CNT transfer technology”
(Adapted from Lingbo etal (2006))
But such advantages of the chemical vapor deposition method are greatly overshadowed by the associated shortcomings of the method. This can be understood from the following statement: “However, the CVD technique suffers from several drawbacks. One of the main challenges for applying CNTs to circuitry is the high growth temperature (>600 °C). Such temperatures are incompatible with microelectronic processes, which are typically performed below 400-500 °C in back-end-of-line sequences. Another issue is the poor adhesion between CNTs and the substrates, which will result in long term reliability issues and high contact resistance. To fabricate microelectronic devices that incorporate CNT blocks, the CNTs should be selectively positioned and interconnected to other materials such as metal electrodes or bonding pads. The common practices for CNT growth on such substrates involve the deposition catalysts such as Fe or Ni on metal layers such as Ti or Ti/Au. Unfortunately, the electrical contact is not necessarily improved because the catalyst nanoparticles are capped by the nanotubes”. To eliminate the above mentioned shortcomings the CNT transfer technology has been suggested. The major advantage of this technology is that it generates structures of CNT whose ends are open.Lingbo et al. (2006)
The Fowler-Nordheim equation
This equation is nothing but the plot between ln(I/V2) and 1/V. If the plot is a straight line, then
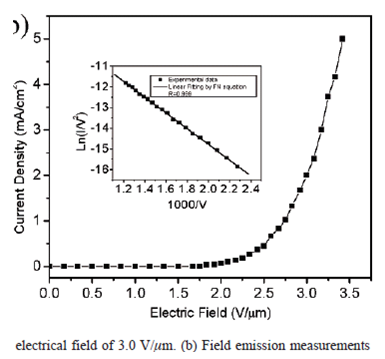
Fowler-Nordheim plot; Adapted from Lingbo et al (2006)
it can be said that the process is in consistence with the Fowler-Nordheim (FN) equation.
This consistency with the Fowler-Nordheim (FN) equation shows that the charge is generated from the process of field emission. Also the value or standard of the fit with the Fowler-Nordheim expression shows the superiority of the contact between the substrate and the nanotube by means of electrical charges. The drop in voltage is also small and also the FN plot is used to assess the factor of enhancement. The Fowler-Nordheim equation is as follows:
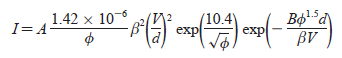
Where
“ I is the current that is emitted (A),
A is the area of emission (m2),
V is the voltage applied,
d is the distance between the CNT tips and the anode (m),
f is the work function (eV),
B is a constant (6.44 _ 109, VeV-1.5m-1).
On plotting ln(I/V2) versus 1/V, the inclination of this linear formulation is given by -B f 1.5 d/â.
Assuming that the work function is 5.0 eV25 the derived field enhancement factor is calculated to be 4540, which is sufficient for application in field emission displays”. Bonard et al (2003).

Researchers to mentor-We write your Assignments & Dissertation
With our team of researchers & Statisticians - Tutors India guarantees your grade & acceptance!
About serviceTitanium Oxide Nanotubes
Introduction
With versatile applications in the technology field like biologically compatible material for implants, ceramic coatings, catalyst etc, it is not surprising that titanium oxides are also being implemented in the field of nanotechnology. In combination with an appropriate semiconductor, it is the major ingredient of the “excitonic photovoltaic cells”. Produced by a method called as anodization, they have a flexible pore size and thickness of the wall. SPIE (2010).
Preparing titanium nanotubes using electrodes
Chemicals used: Pentachlorophenol, Hydrogen fluoride and Nitric acid and a sheet of titanium with a purity of about 99.5% were used.
Preparation of electrode TiO2 nanotubes: After mechanical polishing, the titanium was rinsed in “acid mixture ratio of HF: HNO3:H2O is 1:4:5 in volume”. The electrode was also prepared and the electrolyte possessed “0.2 wt % hydrofluoric acid in water” and a material of platinum was used as a cathode. The color of the surface of titanium should change “from purple to blue, yellow, red, and then finally light red”. Quan.(2005)
Characterization: As stated by Quan et.al (2005), “The morphology, alignment, and composition of the TiO2 nanotubes were characterized using scanning electron microscopy with an accelerating voltage of 20 kV. An energy-dispersive X-ray spectroscopy (EDX) was equipped with the scanning electron microscope. The crystallinity of the TiO2 nanotubes was determined by X-ray diffraction (XRD) using a diffractometer with Cu KR radiation”
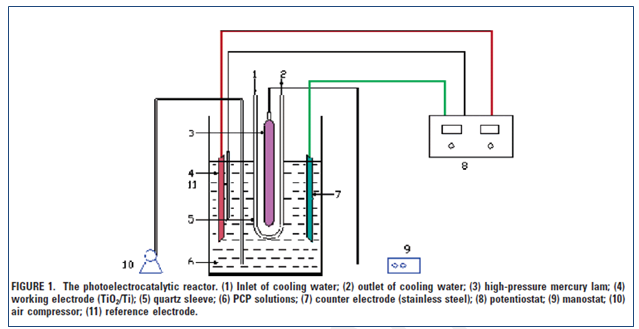
(Adapted from Quan (2005))
Experiment of photoelectrocatalysis: This was done in a single compartment using the ultraviolet radiation, consistent and rapid stirring in the absence of any airflow.
Determination of PCP concentrations: The method of high performance liquid Cromatography was used.
Analysis of TOC: The Total organic carbon analyzer was use to determine the magnitude of mineralization. Quan.(2005).
Manufacture of heat stable titanium oxide nanotubes
Characterization. The characterization of the samples are done with “transmission electron microscopy”, “X-ray diffraction” , “thermal gravimetric analysis” and “Raman spectroscopy”
Test of catalysis: The process of degradation of methyl orange in the presence of water is done in the “Photocatalytic activity experiments”
Hydrogen Titanate nanotube production: A powder of TiO2 is made to disburse in a solution of sodium hydroxide in water and charged into an auto clave and on heating the auto clave in a bath of oil for about twelve hours at a temperature of about 140 degree Celsius. This is then washed in 0.1 M nitric acid solution.
Primary Nanotubes with Titanium possessing sol: As stated by An et. al (2008), “The solution of butyl titanate in absolute ethanol is prepared under vigorous stirring at the volume ratio of 1:15 (solution A). The solution of distilled water, absolute ethanol, and nitric acid (4 mol/L) is prepared at the volume ratio of 1:15: 0.3 (solution B). Solution A is slowly dropped into solution B under continued stirring for 0.5 h (solution C). Then the hydrogen titanate nanotubes are dispersed in solution C under stirring for 12 h. After low-energy sonication for 3.5 h, the product is filtered, dried at 80 °C overnight, and then calcined at 200, 300, 400, and 500 °C, respectively, for 2 h”. Huikin et al. (2008)
Template directed synthesis of nanotubes: This method involves the method of synthesizing the nanotubes using templates. This method was introduced in the 1990s which involves the synthesis of regular sized nanotubes with pores that are cylindrical in shape in length and diameter. There are two types of approaches- the positive templates and the negative templates.
Positive templates: Hwang et. al. has described the process as follows: “Sol–gel-processed TiO2 and Al2O3 nanotubes were also reported via ZnO nanowire templating. Ferroelectric nanotubes such as barium titanate (BaTiO3) and lead zirconate titanate (PZT) were recently demonstrated by depositing thin ferroelectric oxide layers with pulsed laser deposition using Si and ZnO nanowires as positive templates. Electrospun poly(L-lactide) fibers also served as positive templates in order to produce TiO2 nanotubes. Combining the positive template strategy with ALD, the formation of oxide nanotubes with controllable wall thickness has recently been reported by many researchers. Positive templates used were ZnO nanorods, self-assembled polystyrene- block-poly(4-vinylpyridine) block copolymer nanorods, electrospun poly(vinyl alchol) fibers, and tris(8-hydroxyquinoline) gallium nanowires, and Al2O3 nanotubes were prepared by ALD with trimethylaluminum and water as source materials after chemical etching, thermal decomposition, or dissolution of the organic cores”.
Negative Templates:
This uses membranes with pores that give good uniformity in the size, especially in the diameter and length of the tubes that are formed out of titanium. Lakshmi have proposed a method to form “sol–gel titania nanotubes using AAO membranes as negative templates. In their experiments, the formation of oxide walls on the surfaces of inner pores was controlled by the exposure time of titanium isopropoxide precursors Michailowski have also put forward a method to make “highly arrayed TiO2 nanotubes possessing a thin wall layer using a thermal decomposition process with Ti(OiPr)4”. Fisher, Samulski and others have proposed a method of preparing “In2O3 and Ga2O3 nanotubes as well as perovskite nanotubes of BaTiO3 and PbTiO3 by using a sol–gel”. Changdeuck Bae et al. (2008)
Alignment
The importance of alignment in the titianium oxide nanotube can be understood from the following facts. Alignment in the case of titanium oxide nanotubes is important for the development of devices related to photo electricity, electrochemical sensing and phocatalysis. They find wide application in the case of solar cells that have been sensitized using a dye and also in organic and inorganic solar devices. In such cases the use of electrolytes that are liquid in nature leads to serious problems like the leakage. One of the solutions proposed to overcome this problem of leakage is the polymeric sensitizers as the material of transport through the hole so that a completely solid solar cell can be developed. Thus titanium oxide is a good substitute for “1-(3-methoxycarbonyl) propyl-1-phenyl-[6,6]-methanofullerene (PCBM) in the polymeric solar cell to give organic as well as inorganic hybrid solar cells.” Wang et al. (2009)

Perfectly aligned nanotubes
(Adapted from Wang etal (2009))
Method to fabricate single walled nano tubes
A single step process has been developed in order to facilitate the alignment and fabrication of single walled nanotubes (SWNTs) on substrates like the ITO glass. A magnetic field is used to vertically align the nanotubes and the alignment could be fixed in place by directly evaporating the films. The factors that potentially impact the alignment of the nanotubes are the thickness of the film, the rate if evaporation as well as the strength of the field of magnetism. A 200 gauss field was required for the proper alignment. The limit of thickness of the wall of titanium should be between 10 and 100 nm. A thickness below and above this thickness would not properly align the tubes properly.
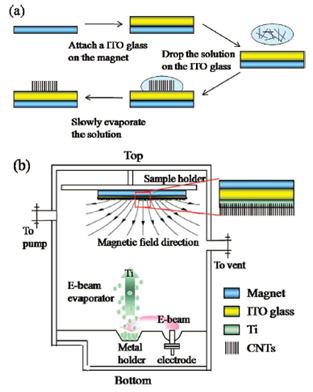
Fabrication of CNTs
(Adapted from Sang et al (2008))
Thus the formation of such vertically aligned single walled nanotubes “affords the alignment of high-aspect-ratio”isolated SWNTs at room temperature without the need for organic binders or further aligning procedures.” There are also many advantages associated with this method like “long-time stability, mild temperature conditions, and good reproducibility”.
There is also lot of studies in the pipeline to develop similar and appropriate single step methods for the subsequent vertical alignment as well as the fixation by evaporation. Sang et al. (2008)
Applications
The potential uses of the nanotubes include its usage in the purification of waste water and the wastes generated by the process of photocatalysis. It is also involved in the splitting process of waste water to generate oxygen and hydrogen and also in the process of organic synthesis.
Titanium oxide nanotubes for delivering drugs
Transfer of the therapeutic drugs into the nanoparticles would greatly increase the efficiency of the delivery of drugs. Materials like “colloidal-templated spheres, macro porous capsules, micelles, liposomes, and dendrimers” are the major particles used for this purpose. In the biological system, certain proteins called opsonins attach to the surface of the particles so that they are recognized by the immune system and cleared easily. Another important benefit of the nanoparticles is that it is easy to synthesize hydroxyl materials into them using simple surface chemistry techniques. Also a number of derivates and hydrophobic tails can be attached to them that facilitate a variety of “host–guest reactions” and also in the designing of drugs in the treatment of cancer. Changdeuck Bae et al. (2008).
Nanotubes as solar cells sensitized to cells: They are used in the process of harvesting the electrons and in transporting materials in the normally used dye sensitized solar cells. These cells also have unique surface areas that have an approximate roughness of about 100. There are a lot of factors that limit the performing efficacy of the dye sensitized solar cells. The efficiency of a solar cell towards the process of photoconversion is given by “? = (FF|JSC|VOC)/Pin, where FFis the fill factor, |JSC| is the absolute value of the short-circuit current, VOC is the open-circuit voltage, and Pin is the incident-light power density”. In order to enhance the efficient performance of dye sensitized solar cells, the area of the surface should be greatly enhanced Changdeuck Bae et al. (2008).
Other potential applications
The properties of the nanotubes that offer a particular advantage to these in versatile fields are “relatively large specific surface area, high aspect ratio structure, and transparent property of oxide nanotubes”. Also the “template directed R-Fe2O3” could be utilized to gas sensors and ion batteries employing lithium Changdeuck Bae et al. (2008).
Diameters and lengths of the nanotubes
The lengths and the diameters of the nanotubes play a significant role in determining their efficiency. Intricately ordered arrays of nanotubes with “tube diameters that range from 55 to 3007 nm and lengths of up to 360 µm8 can be easily fabricated through a simple electrochemical process on titanium foil”. Lily peng et al (2009). The voltage of anodization plays an important role in the determination of the diameter and the length of the tubes is controlled by the quantity of the electrolytes and the time taken for anodization. Thus such nanotubes are known to exhibit increased applications in the field of biology as they show less immune cell activation and also demonstrate the capability to determine the fate of the stem cells. Ainslie et al (2008)
Reference
Ainslie, K. M.; Tao, S. L.; Popat, K. C.; Desai T. A. J. (2008)Biomed. Mater. Res., Part A.
Bonard, J. M.; Klinke, C.; Dean, K. A.; Coll, B. F. (2003). Phys. ReV. B, 67, 115406
Changdeuck Bae et al. (2008). Template-Directed Synthesis of Oxide Nanotubes: Fabrication, Characterization, and Applications. Chem. Mater., 20, 756–767
Choi, W. B.; Jin, Y. W.; Kim, H. Y.; Lee, S. J.; Yun, M. J.; Kang, J. H.; Choi, Y. S.;
Park, N. S.; Lee, N. S.; Kim, J. M. (2001)Appl. Phys. Lett., 78, 1547.
Fowler, R. H.; Nordheim, L. W. (1928) Proc. R. Soc. London, Ser. A, 119, 173.
Gao, B.; Yue, G. Z.; Qiu, Q.; Cheng, Y.; Shimoda, H.; Fleming, L.; Zhou, O. (2001) AdV. Mater., 13, 1770.
Huiqin An, et.al. (2008).Synthesis and Characterization of Thermally Stable Nanotubular TiO2 and Its Photocatalytic Activity. J. Phys. Chem. C, 112, 18772–18775
Lily Peng et al. (2009). Long-Term Small Molecule and Protein Elution from TiO2 Nanotubes. nano letters .No. 5 1932-1936.
Lingbo Zhu et.al. (2006 ). Well-Aligned Open-Ended Carbon Nanotube Architectures: An Approach for Device Assembly nano letters. No. 2 243-247
Okotrub A.V et.al. (2010)Composites Based on Polyaniline and Aligned Carbon Nanotubes. Polymer Science, Ser. B. Nos. 1–2, pp. 101–108
Quan et al. (2005).Preparation of Titania Nanotubes and Their Environmental Applications as Electrode. Environ. Sci. Technol., 39, 3770-3775
Sang Cheon Youn et al. ( 2008)Vertical Alignment of Carbon Nanotubes Using the Magneto-Evaporation Method.
SPIE. 2010. Titanium oxide nanotubes. Retrieved online on march 4 from http://spie.org/x25474.xml?ArticleID=x25474
Sung Mi Jung, Joeoong Hahn, Hyun Young Jung, and Jung Sang Suh. (2006). Clean Carbon Nanotube Field Emitters Aligned Horizontally. Nano Lett. (7), pp 1569–1573
Wang D etal. (2009). Highly Flexible Coaxial Nanohybrids Made from Porous TiO2
Nanotubes . Acsnano. 1249–1257.
What are carbon nanotubes?(2010). Scientific American. Retrieved online on march 4 from: http://www.wisegeek.com/what-are-carbon-nanotubes.htm

Full Fledged Academic Writing & Editing services
Original and high-standard Content
Plagiarism free document
Fully referenced with high quality peer reviewed journals & textbooks
On-time delivery
Unlimited Revisions
On call /in-person brainstorming session
More From TutorsIndia
Coursework Index Dissertation Index Dissertation Proposal Research Methodologies Literature Review Manuscript DevelopmentREQUEST REMOVAL



